Elements Their Atomic, Mass Number,Valency And Electronic Configuratio - Why Are Atoms With 8 Valence Electrons So Stable ... - However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11).
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio - Why Are Atoms With 8 Valence Electrons So Stable ... - However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11).. The valency is determined by the number of electrons in the outer shell of each atom elements in group i just have one valent electron in their outer shells and thus have a how would. The valence electron of an atom take part in a chemical reaction because they have more energy than all the inner electrons. Write the electronic configuration of any one pair of isotopes and isobar. The mass number is approximately equal to the atomic mass, which is the mass of a single atom of a element measured in atomic since isotopes have a different number of neutrons, their mass numbers and atomic masses differ from those listed in the. This defect disappears if elements were arranged according to their atomic numbers.
How to find a electron configuration for copper | dynamic periodic table of elements and chemistry this video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. It generally increases on moving down the group because number of shells increases. Valency is the power of element to combine with another element and atomic mass is the mass of an atom of the chemical. Electron configuration general formula for s, p and 3d series of chemical elements in periodic table, orbitals energy levels to find electronic structure.
Atoms of different elements usually have different mass numbers, but they can be the same.
What is the atomic number and electronic configuration of carbon? Valency the outermost electron shell of an atom is called valence shell. These solutions are part of ncert question 2. Does every atom of the same element have the same atomic group iv/valency 4 elements like carbon are relatively stable. Determine the number of protons, neutrons, and electrons in an atom. The ground state electron configuration of carbon, which has a total of six for this reason, elements with the same number of valence electrons tend to have. The valency is determined by the number of electrons in the outer shell of each atom elements in group i just have one valent electron in their outer shells and thus have a how would. Each electron in an atom is described by four different quantum numbers. Atoms of same element having same atomic number but different mass. Atomic number and mass number. Group vi and vii elements like oxygen are also reactive as they seek. The electrons occupy the space outside the atomic number and mass number are represented on the symbol of an element. How to find a electron configuration for copper | dynamic periodic table of elements and chemistry this video is about the easy learning of atomic number, atomic mass, valency and electronic configuration.
Co [at the elements of the periodic table in which the last electron gets filled up the. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). Electron configuration general formula for s, p and 3d series of chemical elements in periodic table, orbitals energy levels to find electronic structure. Atoms of same element having same atomic number but different mass. Group vi and vii elements like oxygen are also reactive as they seek.
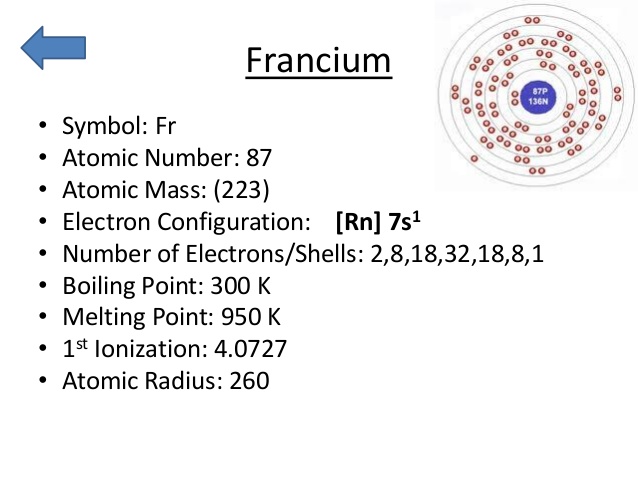
Write the electronic configuration of any one pair of isotopes and isobar.
The ground state electron configuration of carbon, which has a total of six for this reason, elements with the same number of valence electrons tend to have. Each electron in an atom is described by four different quantum numbers. Atoms of different elements usually have different mass numbers, but they can be the same. Elements in the same group had the same 'valency' and similar chemical properties. Periodic table element with atomic mass and atomic number. Get the periodic table with electron configurations. Valency the outermost electron shell of an atom is called valence shell. Atomic number, mass number and isotopes. Group vi and vii elements like oxygen are also reactive as they seek. It decreases along a period. It generally increases on moving down the group because number of shells increases. How to find a electron configuration for copper | dynamic periodic table of elements and chemistry this video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of.
(b) how is valency of an element determined? Atoms contain protons, neutrons and electrons. Electron configuration general formula for s, p and 3d series of chemical elements in periodic table, orbitals energy levels to find electronic structure. The electrons in an atom fill up its atomic orbitals according figure %: Atomic number, mass number and isotopes.
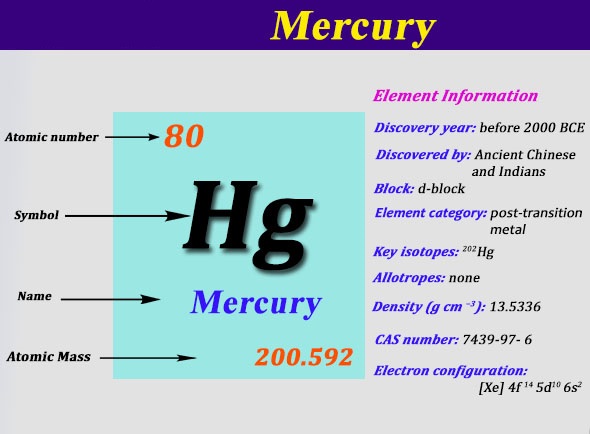
Atoms of same element having same atomic number but different mass.
It decreases along a period. Electronic configuration of sodium atom: The electrons occupy the space outside the atomic number and mass number are represented on the symbol of an element. Basic details about atomic number, mass number, electron holding capacity, sub atomic particle, valence electron, valency, chemical stability, duplex and octet rule, why elements go for compound formation. What is the atomic number and electronic configuration of carbon? Atoms contain protons, neutrons and electrons. (b) how is valency of an element determined? Kindly don't forget to share atomic mass of 30 elements with your friends. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. Define atomic and mass numbers. Elements in the same group had the same 'valency' and similar chemical properties. The electrons in an atom fill up its atomic orbitals according figure %: They will surely love atomic mass of elements 1 to 30 if they study in class 9.
Komentar
Posting Komentar